Abstract
Background Chimeric antigen receptor T-cell therapy (CAR-T) is effective against relapsed and refractory B-cell lymphomas, although durable remissions are achieved in only 30-40% of patients. One proposed mechanism of CAR-T resistance is T-cell exhaustion, mediated in part by upregulation of PD-1 and PD-L1 checkpoint proteins in the lymphoma environment. As such, checkpoint inhibitor (CPI) therapy with anti-PD-1 or anti-PD-L1 antibodies following CAR-T relapse may reverse the exhausted T-cell state and restore CAR-T function. The impact of CPI therapy on response rates, toxicity, and survival after CAR-T merits study in a large real-world cohort.
Methods We conducted a multicenter retrospective review of adult patients with B-cell lymphomas who received CAR-T prior to 2021 and subsequently received any CPI. Primary endpoints were overall response rate (ORR), complete response (CR) rate, progression free survival (PFS), and overall survival (OS) from the time of CPI initiation. Secondary endpoints were univariable analyses of variables associated with inferior OS after CPI initiation. Time-to-event endpoints were analyzed by Kaplan-Meier methodology, and univariable hazard ratios were estimated by Cox proportional hazard models.
Results 96 patients from 15 U.S. academic centers met eligibility criteria. Clinical features included median age 56 years (range 18-79), 32% female, 7% CNS involvement at diagnosis, and 50% primary refractory disease. Most patients had DLBCL (53%), followed by transformed lymphomas (21%), high-grade B-cell lymphoma (9%), primary mediastinal B-cell lymphoma (8%, PMBCL), T-cell/histiocyte-rich large B-cell lymphoma (6%), and follicular lymphoma (2%), with 29% double expressor (DEL) and 17% double hit lymphomas (DHL). Patients received a median 3 lines of therapy before CAR-T (range 1-10) and 28% had prior transplantation. Most patients received axicabtagene ciloleucel (53%), followed by tisagenlecleucel (26%) and lisocabtagene maraleucel (19%). ORR to CAR-T was 57% (25% CR) with median time to recurrence (TTR) after CAR-T of 83 days. After CAR-T, 85% were early relapsers (≤180 days) and 15% were late relapsers (>180 days).
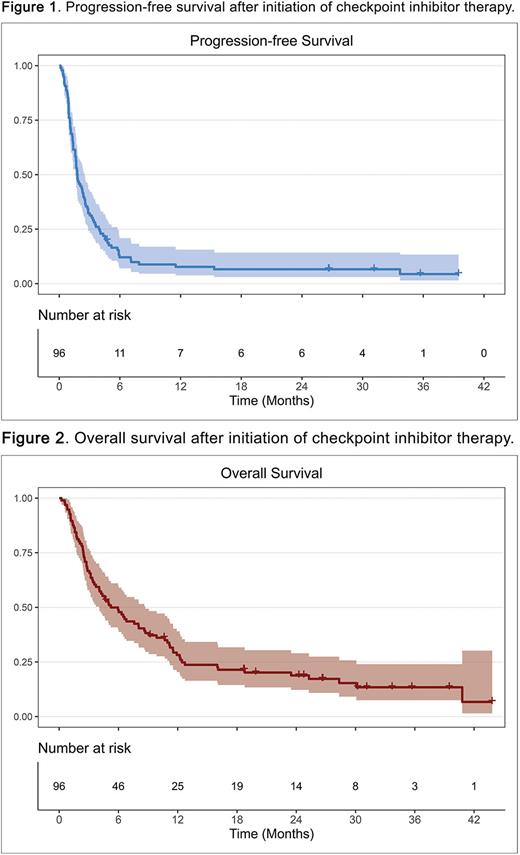
Clinical features at relapse after CAR-T included 82% ECOG PS 0-1, 75% stage III/IV disease, 27% bulky disease, 58% elevated LDH, 42% >1 extranodal site, and 10% CNS involvement. Most patients received pembrolizumab (49%), followed by nivolumab (43%), and 29% received concurrent therapies with CPI, mostly other immunotherapies and lenalidomide. Median CPI cycles received was 3 (range 1-41). Median follow-up after CPI initiation was 152 days (range 7-1333). ORR to CPI therapy was 19% (10% CR), with median duration of response of 7.5 months. Responders to CPI were more likely to have PMBCL (odds ratio 12.5, 95% confidence interval [CI] 2.5-75.5), with no difference in ORR between CAR-T products or early (ORR 16%) versus late relapsers (ORR 36%) after CAR-T. ORR in PMBCL was 63% compared to 12% in DLBCL, and there was no difference in ORR by cell of origin, DEL or DHL in DLBCL. Median PFS and OS after CPI initiation were 55 and 159 days, respectively (Figures 1 and 2). Grade ≥3 adverse events due to CPI occurred in 19% (2% Grade 5). A total of 80 patients died (83%), most commonly of progressive disease (76%).
Factors impacting OS after CPI included grade ≥3 ICANS during CAR-T (HR 2.3, 95% CI 1.2-4.3) and administration of concurrent chemotherapeutics with CPI treatment (HR 0.56, 95% CI 0.34-0.92). No differences in PFS or OS were seen after CPI by histology or CAR-T product. TTR after CAR-T predicted survival after subsequent CPI: median post-CPI PFS and OS were 51 and 131 days in early relapsers versus 128 and 387 days in late relapsers, respectively (p=.008 and .009).
Discussion In this large real-world, multicenter cohort of patients treated with CPI following CAR-T relapse, response rates and median survival were very poor, particularly for patients with early CAR-T relapse. Durable remissions to CPI were rare (5%), and only patients with PMBCL showed improved response rates to CPI but without improvement in OS. Significant improvement in OS in patients receiving CPI concurrently with other therapies may warrant additional study to identify potential synergistic combinations. Finally, these data confirm the dismal prognosis of patients with refractory or early relapsing lymphoma after CAR-T and a pressing need for effective therapeutic approaches.
Disclosures
Yu:AbbVie: Current Employment. Kamdar:Beigene: Consultancy; ADC Therapeutics: Consultancy; Adaptive Biotechnologies: Consultancy; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy; AbbVie: Consultancy; Novartis: Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Research Funding; ImpactBio: Consultancy; Seagen: Speakers Bureau. Haverkos:Viracta Therapeutics: Consultancy; Bristol Myers Squibb: Research Funding. Godfrey:Merck: Research Funding; Secura Bio: Research Funding. Voorhees:Incyte: Research Funding; AstraZeneca: Research Funding. Oliai:Pfizer: Research Funding; Orca Bio: Research Funding; Jazz Pharmaceuticals: Research Funding; Arog: Research Funding; Seagen: Research Funding. Romancik:AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Winter:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; OncLive: Honoraria; Seagen, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees. Hill:Novartis: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; BMS: Consultancy, Honoraria, Research Funding. Villasboas:Aptose: Research Funding; CRISPR: Research Funding; Enterome: Research Funding; Epizyme: Research Funding; Kite Pharma: Research Funding; Regeneron: Research Funding. Karmali:Genentech/Roche: Consultancy, Other: Advisory Board; Kite: Consultancy, Other: Advisory Board, Research Funding, Speakers Bureau; Morphosys/Incyte: Consultancy, Other: Advisory Board, Speakers Bureau; Calithera: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; AstraZeneca: Other: Advisory Board, Speakers Bureau; Eusa: Consultancy; Karyopharm: Consultancy; BMS/Celgene: Consultancy, Research Funding; Pharmacyclics: Consultancy, Other: Advisory Board; Takeda: Research Funding; BeiGene: Consultancy, Other: Advisory Board, Research Funding, Speakers Bureau. Stephens:Epizyme: Consultancy; CSL Behring: Consultancy; Beigene: Consultancy; Lilly: Consultancy; Genentech: Consultancy; Celgene: Consultancy; AstraZeneca: Consultancy; JUNO: Research Funding; Novartis: Research Funding; Mingsight: Research Funding; TG Therapeutics: Consultancy; AbbVie: Consultancy; Newave: Research Funding; Karyopharm: Research Funding; Arqule: Research Funding; Acerta: Research Funding. Schatz:WCG - ACI Clinical: Consultancy. Bachanova:Citius Pharma: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; FATA Therapeutics: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Karyopharma: Consultancy; Gamida Cell: Membership on an entity's Board of Directors or advisory committees, Research Funding. Singh:Rivervest Ventures: Consultancy; Novartis: Patents & Royalties. McGuirk:Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Nextar: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria; Allovir: Consultancy, Honoraria, Research Funding, Speakers Bureau; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Orca Bio: Research Funding; Sana: Honoraria; CRISPR Therapeutics: Consultancy; In8bio, Inc.: Other: IIT Clinical Trial. Bishop:Triumvira: Research Funding; Immatics: Research Funding; Autolus: Consultancy, Research Funding; Arcellx: Consultancy, Research Funding; WindMIL Therapeutics: Consultancy; Bluebird Bio: Consultancy; Iovance: Consultancy; CRISPR Therapeutics: Consultancy, Research Funding; Agios: Consultancy, Honoraria, Other: Travel support, Speakers Bureau; Bristol Myers Squibb: Honoraria, Other: Travel support, Speakers Bureau; Novartis: Consultancy, Honoraria, Other: Travel support , Research Funding; Sanofi: Honoraria, Speakers Bureau; Celgene: Honoraria; Incyte: Honoraria, Other: Travel support , Speakers Bureau; Chimeric Therapeutics: Consultancy; Tmunity: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel support, Research Funding, Speakers Bureau; Sana Biotechnology: Consultancy; ADC Therapeutics: Speakers Bureau; Servier: Speakers Bureau. Riedell:Sana Biotechnology: Consultancy; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Intellia Therapeutics: Membership on an entity's Board of Directors or advisory committees; Nektar Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite/Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy; Takeda: Membership on an entity's Board of Directors or advisory committees; MorphoSys: Research Funding; Calibr: Research Funding; Tessa Therapeutics: Research Funding; Fate Therapeutics: Research Funding; Xencor: Research Funding. Kline:iTeos, Merck, Verastem: Research Funding; Karyopharm, Kite/Gilead, Merck, MorphoSys, Seagen, Verastem: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal